
Now, why is this important? Well, anything that is radiating will lose energy because it gives off radiation. Permittivity” constant (a constant of nature that shows up a lot in electrodynamics). Larmor’s power formula tells us how much radiation or more precisely, the power of electromagnetic radiation that is given away and this is given by the following formula: Here, q is the charge of the accelerating charged particle, a is its acceleration, c is the speed of light and ε 0 is the “vacuum So, an electron orbiting the nucleus of an atom is accelerating and therefore also produces electromagnetic radiation. The same thing happens when the Earth orbits the Sun – it is in accelerated motion in order to remain in orbit. This is because for the electron to remain in orbit, its velocity must at least change direction (and not just be a straight line) and therefore, there must be acceleration. The answer can be found in classical electrodynamics and goes by a result called Larmor’s power formula – it describes the phenomenon that accelerating charges produce electromagnetic radiation.Īnything in orbital motion, including an electron orbiting around the nucleus, is accelerating. Because of its charge, any electron in this orbiting type of motion would inevitably fall into the nucleus. However, something special happens because the electron has an electric charge. The original – classical – picture of an atom was of a ball-like electron orbiting the nucleus much like how the moon orbits the Earth.Įven if this orbiting-model were correct, the electron should still not just “fall” into the nucleus – it should keep orbiting, just like the Earth keeps orbiting the Sun and doesn’t just fall into it randomly. In fact, ALL electrons should and we should not have a single stable atom – but for more reasons than your intuition might tell you!

Everything in classical mechanics is governed by Newton’s laws (like F=ma).Īccording to classical mechanics, electrons should indeed fall into the nucleus. Instead, we’ll actually look at what our current understanding of physics and quantum mechanics really predicts and how this comes about from our mathematical models.Ĭlassical mechanics is what we used to describe physics previously, before quantum mechanics was developed. Now, this article won’t be your typical pop-science content you’ll often find regarding this question. This creates a bit of a paradox, but we’ll then take a look at how quantum mechanics changes things and fixes this.Īlso, this new quantum mechanical arena allows for more phenomena, such as an electron being found within and interacting with the nucleus, allowing effects such as electron capture. In this article, we’ll discuss the classical picture of an electron and see why, according to the classical rules like Newton’s laws of motion, electrons should fall into the nucleus.

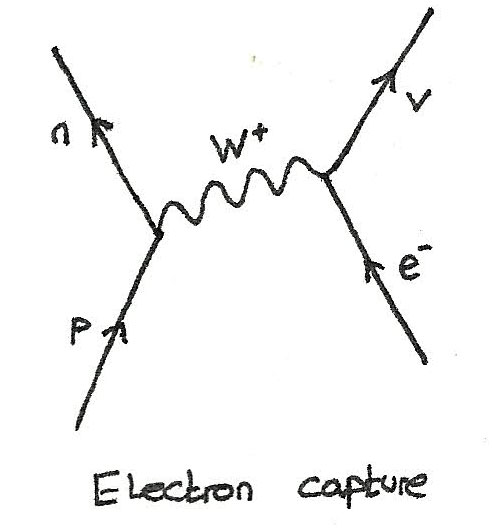
However, there is nonetheless a small probability to find an electron inside the nucleus, which results in phenomena like electron capture. So why exactly do electrons not fall into the nucleus?Įlectrons obey the rules of quantum mechanics, according to which they can only have very specific energies and therefore cannot fall into the nucleus. However, since you’re reading this and you’re made out of stable atoms and so is every other object you see around you, there must be something else going on. In this case a proton loses its positive charge therefore it is carried away to the electron via the W + boson transforming the electron into an electron-neutrino.If electrons are negatively charged and the nuclei in atoms are positively charged, they should attract each other and intuitively, it would seem that electrons should just fall into the nucleus. The electron-neutrino in this diagram is drawn with a shallow gradient to show that it travels close to the speed of light.įinally the boson mediating the interaction is added as a wave between the two points of interaction. It should be recognised that baryon conservation means a baryon becomes another baryon and lepton conservation means that a lepton is transformed from one type of lepton into another. The particles after the interaction are drawn at the top of the Feynman diagram. Both the proton and electron in this diagram are drawn with a steep gradient to show their relatively small velocity compared to the speed of light. The particles prior to the interaction are drawn first at the bottom of the Feynman diagram.


 0 kommentar(er)
0 kommentar(er)
